
Pharmaceutical companies are facing more pressure than ever to ensure that their products are safe, effective, and meet regulatory standards.
With the rise of digital technologies, the use of pharma validation software has become increasingly important in streamlining the validation process and reducing the risk of errors. By automating many manual tasks, these software tools enable companies to increase efficiency while reducing costs and errors.
However, not all tools are created equal. In this article, we’ll walk through our unique approach to validation, and show you how it’s been specifically designed to accommodate the complexities of the life science industry.

If you're in the pharmaceutical industry, you know how important it is to have a robust validation process in place. But with so many options out there, it can be hard to know which one to choose.
That's where we come in. We understand the unique needs and challenges faced by pharma companies, and our software has been designed to address those needs head on.
There isn’t a one-size-all approach to drug production. As such, we’ve taken an innovative approach with our software validation platform to ensure it meets the needs of all manufacturing use cases, from preclinical benchtop to large-scale commercial manufacturing.
By leveraging the latest technologies, we've created a user-friendly platform that streamlines the validation process and helps companies ensure compliance with regulatory standards:
Read on to learn four ways we’ve tailored our validation process to meet the demands of the pharmaceutical industry.
Our pharma validation software has been designed from the ground up to meet the demands of the life science industry and provides a tailored solution for companies in this field.
Unlike other platforms which cast a wide net, our solution is custom-tailored to the life sciences. It focuses on risk-based Computer Software Assurance (CSA), as per FDA guidelines, to reduce heavy lifting on your end and accelerate time to market.
To achieve this, we take a three-stage approach to validation every time we release a new update:
This process enables us to streamline updates, ensure accuracy, and provide our users with a hassle-free validation platform so they can focus on batch requirements.
Pharma companies are under more pressure than ever to release safe, effective products. This is often easier said than done, given the extensive costs associated with drug manufacturing, not to mention the time it takes to bring therapeutics to market.
That’s where our Tempo Cloud Quality Program comes in — it moves away from traditional Computer Software Validation (CSV) in favor of CSA.
Most notably, it flips the 80/20 rule on its head! With the Tempo Cloud Quality Program, you can go from 80% documentation and 20% testing to 80% testing and 20% documentation. Talk about a huge win in highly regulated industries like pharma.
“A common misconception about risk is that avoidance is the only option. The greater the risk, the greater the reward. Let’s mitigate, reduce, and accept the risk — in order to take advantage of the great reward.”
— Michael Martone, Director, Quality Systems, Apprentice
With a reputable process that allows for agile development, we’re able to effectively and efficiently develop new features that meet Good Practice (GxP) quality standards:
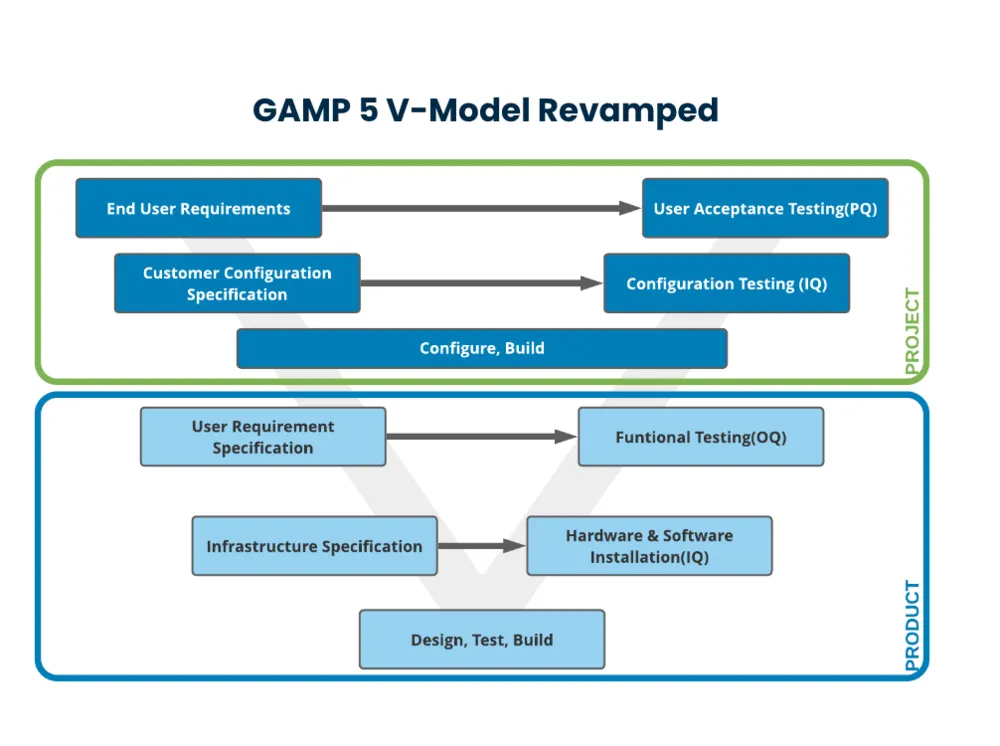
Our GAMP 5 V-model starts with the user requirements to inform the steps and documentation throughout the process. It then splits the model into two perspectives:
Our methodology incorporates validation into implementation. To achieve this, we take a seven-step approach:
Throughout this 7-step process, our goal remains the same: to provide you with a seamless experience as we streamline your software validation processes.

We formed our three-prong approach to validation after considering the complex needs of pharma organizations and working alongside industry stakeholders.
Read on to see how our process takes place over the three stages outlined below.
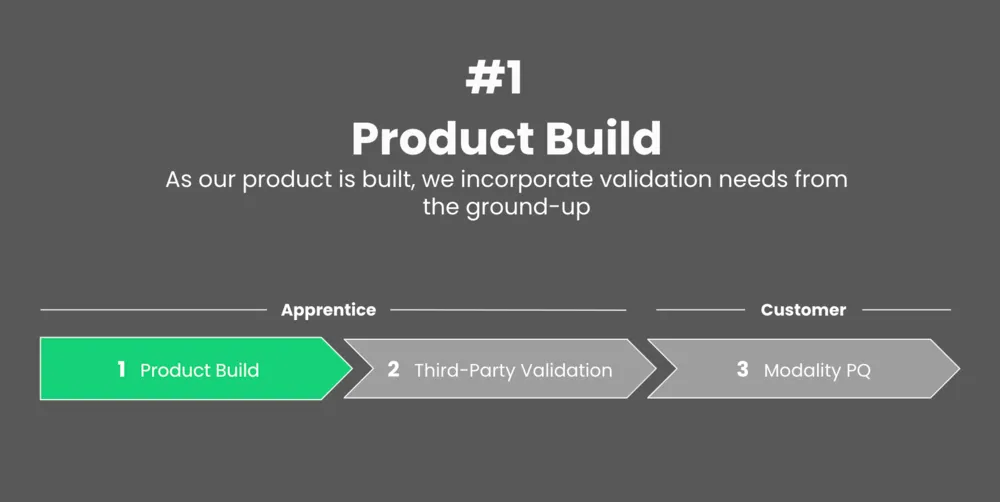

With our solution, validation starts right at the beginning: with production development.
Our SDLC factors in the testing and documentation needed for Installation Qualification (IQ) and Operational Qualification (OQ).
Take a look at the Apprentice product development methodology:
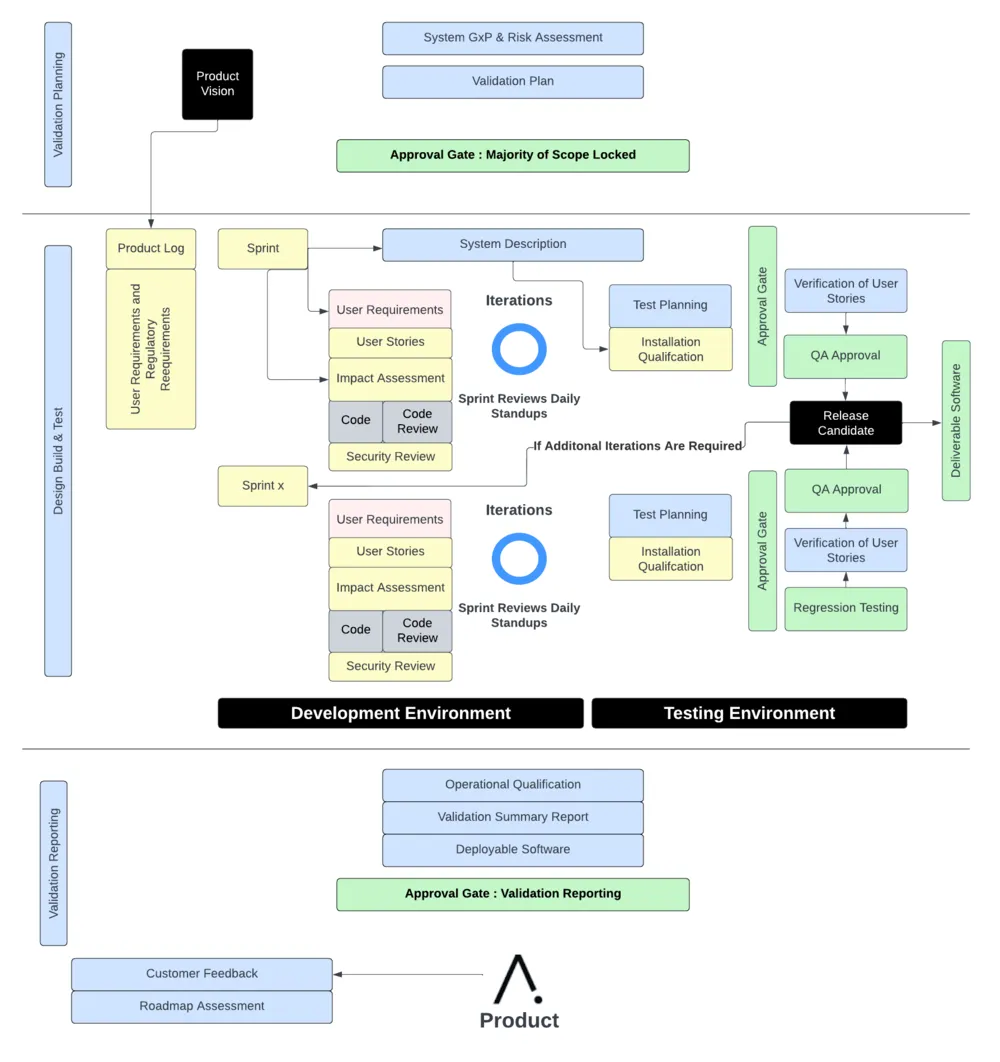
In particular, we focus on software hazard analysis and validation testing and documentation:
Our SDLC facilitates validation from the start, so you can rest easy knowing that all of your documentation and processes are compliant.
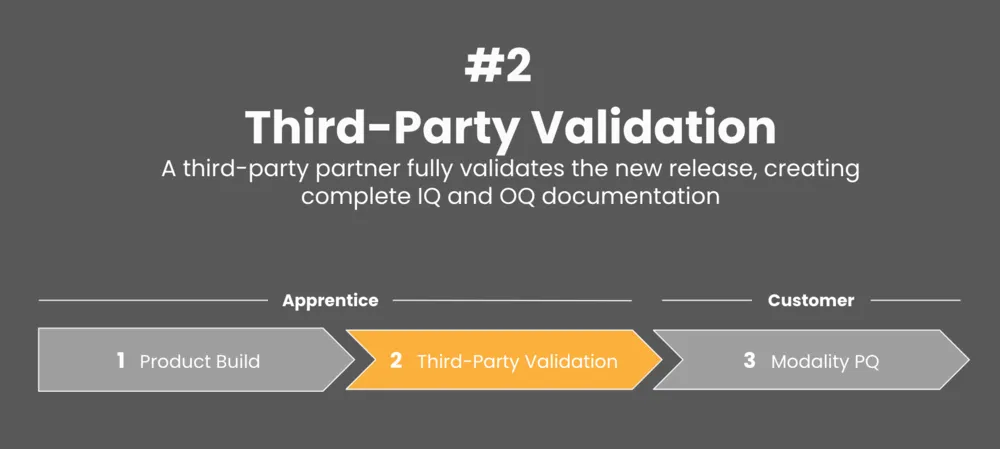

Release validation is a key step in the drug production process.
To ensure success, we’ve partnered with CompliancePath for third-party validation purposes.
CompliancePath is a veteran of the life science industry and a global leader in Software Assurance across the United States and Europe. Additionally, the company also hosts and conducts countless audits across the GxP disciplines including ISO13485 (inclusive of IEC62304/ISO14971), ISO27001, SOC 1 & 2, EHNAC, and FEDRAMP.
Together, we work to ensure that users are ready for operations and audits with an independent partner. Our platform provides an objective review from a neutral industry expert to help you align with the foundational guidance provided by the FDA.

“Self-validation is extremely difficult. When possible, an independent evaluation is always better, especially for high-risk applications.”
— U.S. Food and Drug Administration
With each validated release, CompliancePath validates Tempo’s core functionality by:
This checks-and-balances process allows users to accelerate PQ and focus testing efforts on critical elements while deploying Tempo faster.
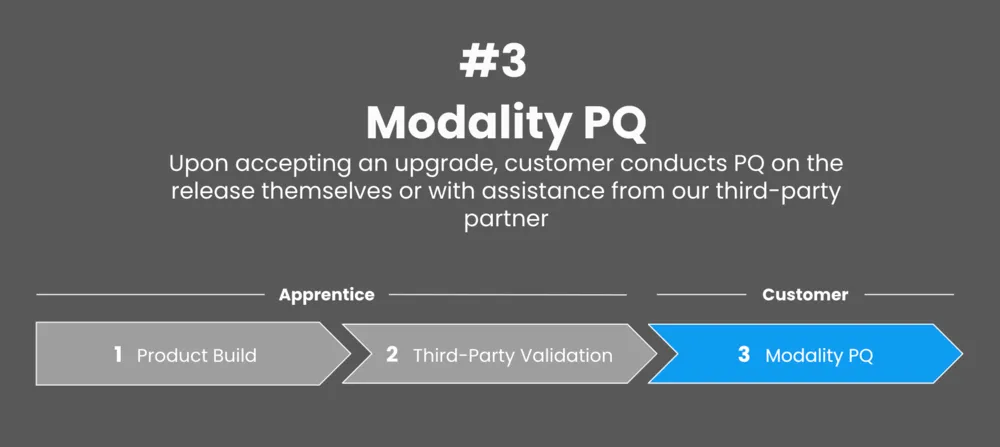

To determine the best PQ process, we partnered with a top 3 biotech organization to develop, test, and prove our approach.
We started by selecting a representative sample of procedures, based on a modality from across their product portfolio. We then identified the standard to PQ that every other procedure of that modality would derive from.
Finally, we assessed the critical steps within these standards and verified the efficacy of them through the successful completion of said steps.
Ultimately, we determined that procedures derived from the standard no longer require PQ and any new variations only need to test the changed steps, as well as any steps affected downstream.
Therefore, each user must use a trusted third-party partner such as CompliancePath to validate their individual configuration of the Tempo platform. This step ensures that the platform functions as expected for their specific needs.
Michael Martone is committed to quality. As Director of Quality Systems at Apprentice, he keeps Quality priorities top of mind and on target for our teams and our clients.
Read on to learn more about Michael’s background and industry insights on pharmaceutical software validation.
Michael Martone brings over a decade of experience to his tenure at Apprentice. Michael covers a wide range of process areas in his work, providing guidance on Quality as well as Compliance and Validation.
Michael’s experience spans a number of industries, including Life Science, Medical Device, Aerospace, and Defense. He provides consulting across the U.S. and has implemented Management Systems for adherence to ISO9001, ISO13485, ISO27001, ISO17025, ISO62304, and SOC2.
Ultimately, Michael’s career mission comes down to a single goal: educating organizations on the crucial roles played by Quality, Compliance, and Validation. In this way, he continually demonstrates the value of Quality as a critical source of organizational enablement.
“My top recommendation for organizations looking to achieve a successful validation is to embrace change. The industry has shifted to accepting risk and thinking critically — we must maximize on these methodologies to bring drugs to the patients who need them faster.”
Software validation is no joke — the process is not only time consuming, but it’s also crucial to ensure regulatory compliance. That’s why it’s no place for shortcuts.
Our software is purpose-built for pharma’s specific needs and stringent security requirements, as opposed to being a general-purpose manufacturing software that’s adapted to a pharma use case. As a result, when you use Tempo you’ll have peace of mind knowing you’re using a system that’s secure, compliant, and tailored to your needs.
Want to learn more about Tempo’s software validation process? Book a call with a member of our team to find out more.


